Breakthroughs in Radiopharmaceutical Drug Conjugates (RDCs)
Radiopharmaceutical Drug Conjugates (RDCs) are emerging as a cutting-edge approach in precision oncology, integrating targeted therapy with radiopharmaceuticals to selectively eliminate cancer cells while minimizing damage to normal tissues. In recent years, FDA approvals of 177Lu-PSMA-617 (for prostate cancer) and 225Ac-DOTATATE (for neuroendocrine tumors) have accelerated the clinical application of RDCs, highlighting their potential in personalized cancer treatment.
However, RDC development still faces key challenges, including radioisotope stability, targeted delivery efficiency, systemic toxicity management, and scalable manufacturing. To address these limitations, Litchlab provides an innovative, integrated RDC delivery platform that enhances drug stability, precision targeting, and therapeutic efficacy while streamlining clinical translation.
Litchlab’s RDC Delivery Technology: Key Advantages
1. Advanced Nanocarrier Systems for Enhanced Stability & Efficacy
Litchlab employs state-of-the-art lipid nanoparticles (LNPs), polymeric nanoparticles (PNPs), and nanomicelles (NMs) to encapsulate radioisotopes, reducing premature leakage and off-target toxicity while optimizing tumor accumulation.
LNP Encapsulation System:
Compatible with 177Lu, 225Ac, and other α/β-emitting radioisotopes, increasing circulation half-life and tumor retention.
Engineered for pH-responsive drug release, ensuring controlled payload activation within tumor microenvironments.
Polymeric Nanoparticles (PNPs):
PEGylated modifications enhance systemic stability and minimize non-specific organ uptake.
Ideal for ligand-conjugated RDCs, boosting receptor-ligand interactions and tumor cell uptake.
2. Optimized Targeting & Conjugation Chemistry for Superior Delivery
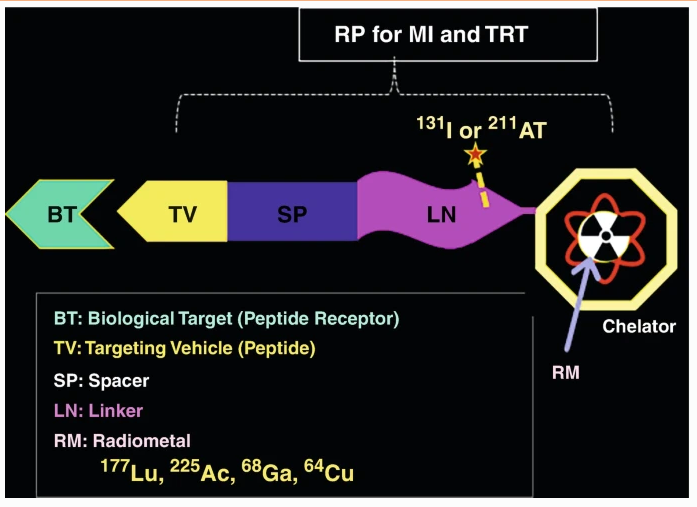
Litchlab’s proprietary radioimmunoconjugate (RIC) technology optimizes RDC targeting through high-affinity chelators and precision bioconjugation strategies.
Enhanced Chelation Chemistry: High-affinity chelators such as DOTA, NOTA, and TETA ensure radioisotope stability (e.g., 225Ac, 177Lu, 90Y), minimizing systemic leakage and toxicity.
Dual-Targeting Strategies: Antibody-Aptamer Conjugates (AACs) improve tumor selectivity, increasing cellular uptake and reducing off-target effects.
pH-Triggered Release Mechanism: Smart linkers ensure controlled payload activation within the acidic tumor microenvironment (pH 6.5-6.8), maximizing therapeutic potency.
3. Theranostic Integration for Personalized RDC Therapies
Litchlab integrates theranostics (therapy + diagnostics) into RDC development, optimizing patient selection and treatment efficacy through real-time molecular imaging.
Ligand-Based RDC Platforms: Tailored for PSMA, SSTR, and FAP receptors, enabling personalized treatment strategies for prostate cancer, neuroendocrine tumors (NETs), and pancreatic cancer.
PET/SPECT Imaging-Guided Therapy:
68Ga-DOTATATE/225Ac-DOTATATE (for neuroendocrine tumors)
68Ga-PSMA-11/177Lu-PSMA-617 (for prostate cancer)
89Zr-Trastuzumab/225Ac-Trastuzumab (for HER2+ breast cancer)
Patients are pre-screened via PET/SPECT imaging to confirm receptor expression, ensuring RDC treatment is precisely targeted to responsive patients.
Litchlab’s Role in RDC Commercialization & Clinical Translation
Litchlab provides a full-spectrum RDC delivery solution, accelerating drug development from early research to GMP-compliant production, facilitating IND/NDA regulatory submissions.
| Technology | Litchlab Solution | Clinical Application |
|---|---|---|
| Nanocarrier RDC Delivery | LNPs, PNPs, nanomicelles | Enhances tumor targeting for α/β-emitting isotopes |
| Optimized Chelators | DOTA, NOTA, TETA | Ensures 225Ac, 177Lu, 90Y stability & safety |
| Precision Targeting | Antibody-aptamer conjugation, ligand-modified RDCs | Improves patient stratification and tumor uptake |
| Theranostics Integration | PET/SPECT imaging + RDC therapy | 68Ga/177Lu, 89Zr/225Ac theranostic approaches |
| Scalable Manufacturing | Microfluidics, high-pressure extrusion, GMP-scale production | Supports clinical & commercial development |
Future Outlook: Advancing RDC Precision Medicine
With the recent success of Datroway (datopotamab deruxtecan) in ADC drug development, precision drug delivery has become a core focus in oncology. RDCs, as the next-generation targeted radiotherapy, demand superior delivery efficiency, safety, and scalability.
Litchlab remains at the forefront of RDC innovation, leveraging its cutting-edge nanocarrier systems, advanced bioconjugation strategies, and theranostic platforms to drive prostate cancer, breast cancer, and neuroendocrine tumor treatment breakthroughs. By partnering with global pharmaceutical leaders, Litchlab is accelerating the future of precision radiopharmaceutical therapies
For more information, please feel free to contact us at:
E-Mail:RD1@Litchlab.com




