Breakthroughs in the RDC Field & Market Trends
The global Radiopharmaceutical Drug Conjugate (RDC) sector is witnessing rapid advancements. On March 25, 2025, Kelun-Biotech, a leading Chinese biopharmaceutical company, announced that its radiopharmaceutical conjugate SKB107 had received clinical trial approval from the National Medical Products Administration (NMPA) for the treatment of advanced solid tumor bone metastases. This milestone highlights the expanding clinical applications of RDCs in oncology (bydrug.pharmcube.com).
Meanwhile, the RDC market continues to surge. Novartis's RDC drugs, Pluvicto and Lutathera, have shown remarkable growth. In 2024, Pluvicto recorded a 42% year-over-year sales increase, reaching $1.392 billion, while Lutathera sales rose by 20% to $724 million (bydrug.pharmcube.com). These figures highlight RDCs as a transformative technology in precision nuclear medicine and oncology therapy.
Litchlab’s Advanced RDC Delivery Technology: Driving Precision, Safety, and Efficacy
As a global innovator in RDC delivery technologies, Litchlab is dedicated to enhancing the stability, targeting efficiency, and delivery precision of radiopharmaceutical drug conjugates, accelerating their transition from lab research to clinical application and commercialization.
Key Technological Advantages of Litchlab’s RDC Platform
1. Nano-Delivery Systems for Enhanced Stability
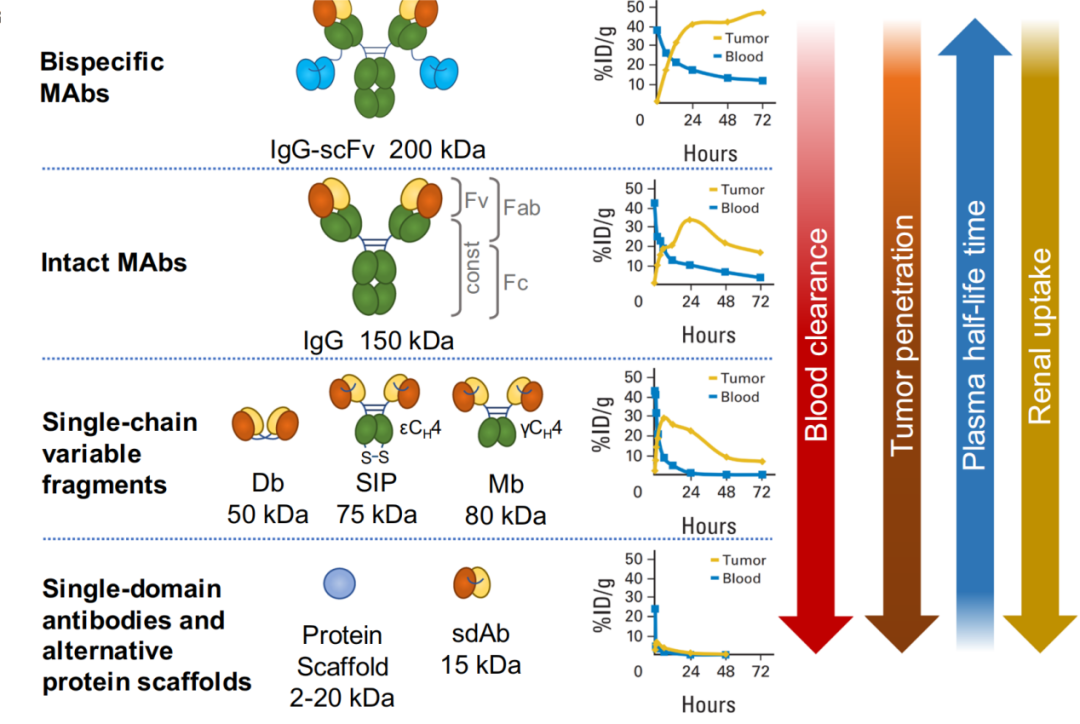
One of the key challenges in RDC development is premature radionuclide release, rapid blood clearance, and potential kidney toxicity. Litchlab’s proprietary nano-delivery systems address these challenges through:
Lipid Nanoparticles (LNPs) for Improved Stability: Encapsulating radionuclides in LNPs enhances their circulation time and prevents non-specific toxicity, improving therapeutic efficacy.
Polymeric Nanocarriers: Utilizing polymeric microspheres (PMs) and polymeric micelles, Litchlab reduces off-target diffusion of radiopharmaceuticals and extends drug half-life.
2. Precision-Targeted Conjugation for Optimal Therapeutic Outcomes
High-Affinity Antibody-Radionuclide Conjugation: Litchlab optimizes antibody-chelator-radioisotope linkage to maximize tumor-specific targeting and improve the bioavailability of RDCs.
pH-Responsive Release Mechanism: Ensuring efficient RDC activation in the tumor microenvironment (pH 6.5-6.8) for precise intracellular drug release and increased anticancer activity.
PEGylation for Extended Circulation Time: Implementing PEGylation strategies reduces rapid clearance and enhances RDC accumulation in tumor tissues while minimizing off-target toxicity.
3. cGMP-Grade RDC Manufacturing for Rapid Commercialization
Litchlab provides cGMP-compliant RDC production capabilities, supporting global partners in RDC development from early-stage R&D to full-scale manufacturing:
Radionuclide Encapsulation & Stability Optimization: Specializing in [225Ac], [177Lu], [89Zr], and [64Cu] encapsulation for enhanced therapeutic stability.
Scalable Production Techniques: Employing microfluidics and supercritical fluid (SCF) technology to improve formulation consistency and industrial-scale reproducibility.
Sterile Formulation & Quality Control: Implementing automated aseptic cGMP production systems to meet global regulatory standards (FDA, EMA, NMPA).
Litchlab RDC Platform: Key Clinical Applications
1. Radioimmunotherapy (RIT) with Radionuclide-Labeled Antibodies
Prostate Cancer: Supporting the development of PSMA-617-177Lu radiopharmaceuticals to enhance targeted tumor irradiation while minimizing hematologic toxicity.
HER2-Positive Breast Cancer: Optimizing HER2-directed radiolabeled antibodies (e.g., [89Zr]-trastuzumab) for high-precision PET/CT imaging, enabling early detection and real-time treatment monitoring.
2. Radiopharmaceutical Nanocarriers for Enhanced Tumor Targeting
Bone Metastases Therapy: Utilizing nanoparticle-based RDCs, such as 177Lu-EDTMP, to improve uptake in bone lesions and reduce systemic toxicity.
Glioblastoma (GBM) Treatment: Developing nanoparticle-RDC systems capable of crossing the blood-brain barrier (BBB) for efficient delivery of radiopharmaceuticals to CNS tumors.
3. Combination Therapy & Personalized Nuclear Medicine
RDC + Immunotherapy: Investigating synergistic effects of RDCs with immune checkpoint inhibitors (PD-1/PD-L1) to enhance tumor immune response.
AI-Driven RDC Precision Therapy: Integrating AI-powered biomarker analysis to optimize patient selection and treatment strategies for personalized radiopharmaceutical therapy.
Partnering for the Future: Driving RDC Innovation Together
As the global RDC market continues its rapid growth, Litchlab is committed to becoming a leading force in radiopharmaceutical drug delivery. By leveraging cutting-edge delivery technologies, cGMP production capabilities, and strategic collaborations, we aim to accelerate the development and commercialization of next-generation RDC therapies, shaping the future of precision nuclear oncology.
For more information, please feel free to contact us at:
E-Mail:RD1@Litchlab.com




