
In early 2025, multiple groundbreaking advancements in vaccine development were reported. GlaxoSmithKline (GSK) announced promising Phase II clinical trial results for its mRNA-based seasonal flu vaccine. Moderna published preclinical data in Cell on its mRNA-1769 candidate for monkeypox, showing superior protection compared to traditional vaccines. Meanwhile, Pfizer and BioNTech released clinical data on their next-generation trivalent mRNA flu vaccine, demonstrating strong immune responses across all targeted strains.
These breakthroughs highlight that vaccine innovation is accelerating rapidly, with effective and safe delivery systems becoming the critical enabler. As a global leader in drug delivery technology, Litchlab is advancing next-generation vaccine delivery platforms to optimize stability, enhance efficacy, and accelerate clinical translation.
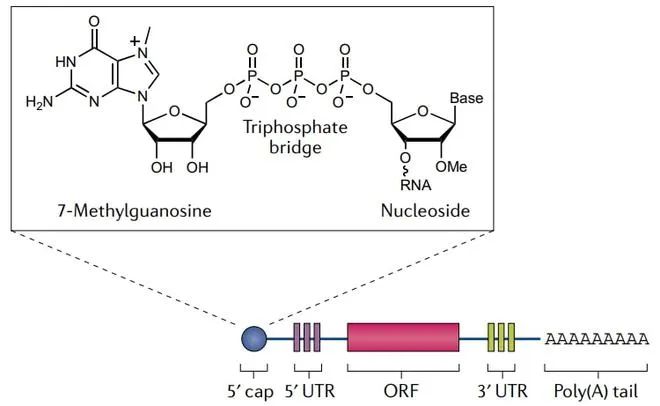
Litchlab has developed state-of-the-art delivery systems for mRNA vaccines, protein subunit vaccines, viral vector vaccines, and DNA vaccines, optimizing delivery strategies for different vaccine modalities. Our core platforms include:
Lipid Nanoparticle (LNP) Delivery for mRNA Vaccines
Polymeric Microsphere (PLGA) Systems for Protein and Subunit Vaccines
Advanced Nanocarriers for Viral Vector and Mucosal Vaccines
Efficient mRNA vaccine delivery requires stable and optimized LNP systems. Litchlab has pioneered LNP innovations to address key challenges:
Optimized Ionizable Lipids: Novel biocompatible ionizable lipids improve mRNA encapsulation, intracellular delivery, and reduce hepatotoxicity.
Targeted Ligand Modification: Overcomes the traditional hepatic accumulation of LNPs by incorporating glycosylation, aptamer, or peptide-based targeting for lymph node and antigen-presenting cell (APC) delivery, enhancing immune activation.
Enhanced mRNA Stability: Optimized lipid membrane composition and RNA modifications increase circulation half-life and antigen expression duration.
Immunogenicity Enhancement: LNP structures promote APC uptake, enhancing both cellular (CD8+ T cells) and humoral (B cell) immune responses for superior vaccine efficacy.
For protein-based, subunit, and virus-like particle (VLP) vaccines, Litchlab’s PLGA microsphere delivery system provides controlled release and enhanced immune activation:
Sustained Antigen Release: Controlled biodegradation allows single-dose administration with staged antigen presentation, reducing the need for multiple booster shots.
Adjuvant Integration for Enhanced Immunogenicity: Co-encapsulation of TLR agonists (CpG, Poly I:C) and QS-21 adjuvants enhances dendritic cell activation, improving vaccine potency.
Enhanced Protein Stability: Protects protein antigens from premature degradation, ensuring effective immune responses in VLP vaccines, recombinant protein vaccines, and glycovaccines.
Litchlab offers cutting-edge solutions for viral vector vaccines and mucosal immunization strategies:
Viral Vector Stabilization: Nano-encapsulation improves the stability of adenovirus, poxvirus, HSV-based vaccines, reducing cold-chain dependence and enhancing delivery efficiency.
Nanoparticle-Based Mucosal Vaccination: Optimized formulations for intranasal and oral vaccines enhance mucosal IgA and systemic immune responses, ideal for COVID-19, RSV, and influenza vaccines.
Biocompatible Nanoparticle Modifications: Fine-tuned surface chemistry improves biodistribution, minimizes off-target inflammation, and enhances vaccine safety profiles.
With its cutting-edge delivery platforms and cGMP-scale production capabilities, Litchlab supports vaccine developers from early-stage research to clinical trials and large-scale manufacturing:
End-to-End mRNA Vaccine Development: From RNA optimization and LNP formulation to in vitro and in vivo delivery assessments.
Customized Targeted Delivery: Tailored solutions for different indications (e.g., cancer vaccines, infectious disease vaccines) to enhance immune responses.
GMP-Ready LNP and PLGA Manufacturing: Fully compliant with global regulatory standards (FDA, EMA, NMPA), facilitating IND filings, clinical trials, and commercial-scale production.
The global vaccine landscape is evolving towards greater precision, efficiency, and safety. Litchlab remains at the forefront of vaccine delivery innovation, collaborating with leading vaccine developers to drive next-generation breakthroughs and accelerate commercialization.
For more information, please feel free to contact us at:
E-Mail:RD2@Litchlab.com