
In the rapidly evolving therapeutic landscape, liposomal drug delivery has emerged as a vital platform for addressing complex diseases—including cancer, infectious diseases, genetic disorders, and immunotherapies. Litchlab, an innovation-driven Contract Development and Manufacturing Organization (CDMO), is at the forefront of this transformation, offering comprehensive solutions across the entire lifecycle of liposomal drug development and production.
By integrating cutting-edge nanotechnology, formulation science, and translational expertise, Litchlab empowers global biopharma partners from early-stage development to GMP manufacturing and IND submission, accelerating their R&D pipelines.
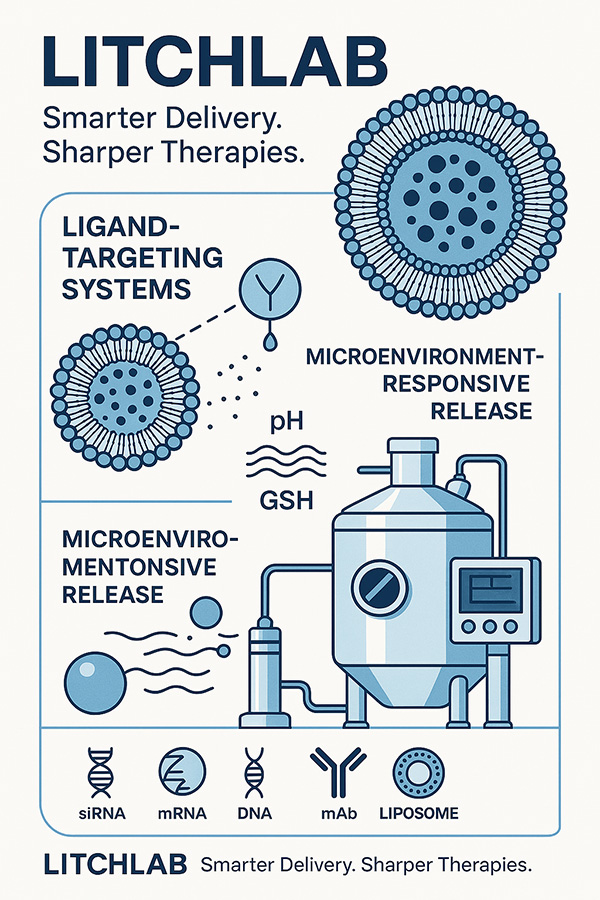
Diverse Ligand Options: scFv, Fab fragments, bispecific antibodies, aptamers, RGD peptides, glycan ligands, CPPs, and more
Target Coverage: HER2, EGFR, PD-L1, PSMA, ASGPR, CXCR4, TfR, folate receptor
PEG Spacer Arm Optimization: Fine-tuned ligand density and orientation for optimal receptor engagement
Dual-Ligand Systems: Designed to address tumor heterogeneity and multi-target synergy
pH-Sensitive Constructs: DOPE/CHEMS-based designs that disassemble in acidic tumor microenvironments
GSH-Triggered Mechanisms: Disulfide linkers enable intracellular release in high-glutathione conditions
Enzyme-Responsive Designs: MMP-2/9 and Cathepsin B-cleavable structures for site-specific delivery
Integrated Multi-Stimuli Designs: Dual triggers such as pH+enzyme or GSH+pH for enhanced spatiotemporal control
High Encapsulation Efficiency (>85%), Particle Size 80–200 nm, PDI < 0.2
Compatible Payloads:
Small molecules (e.g., paclitaxel, irinotecan)
Biologics (e.g., monoclonal antibodies, enzymes)
Nucleic acids (siRNA, mRNA, CRISPR)
Radiolabels (e.g., Lu-177, Zr-89, Ac-225)
Advanced Loading Strategies:
Active loading (pH/ion gradients)
Drug-gene co-delivery systems (e.g., paclitaxel + siVEGFA)
Lipopolyplex RNA delivery
Multiple Production Techniques: Thin-film hydration, REV, microfluidics, high-pressure homogenization
QbD + DoE Modeling: Robust parameter design for consistent batch-to-batch performance
Comprehensive QC Methods: DLS, HPLC, TEM, zeta potential, encapsulation analysis
Lyophilization Support: Freeze-drying process development for improved stability and logistics
GMP-Compliant Manufacturing: Batch production from Phase I to commercialization; equipped with Grade A/B cleanrooms and cold chain systems
Regulatory Dossier Preparation: Expertise across FDA, EMA, NMPA for IND/IMPD CMC modules
Regulatory Compliance: Adheres to ICH Q8/Q9/Q10/Q11, EU Annex 1, USP
Global Tech Transfer Experience: Successfully partnered with multiple biotech firms in US and EU
Litchlab’s CDMO platform supports a broad array of high-impact therapeutic programs:
| Indication | Drug Type | Target Marker | Delivery System |
|---|---|---|---|
| Breast/Ovarian Cancer | Paclitaxel / Doxorubicin | HER2, EGFR | Targeted Liposomes |
| Liver Cancer | Lu-177 / siRNA | ASGPR, PD-L1 | Dual-Responsive Liposomes |
| Respiratory Vaccines | mRNA / Adjuvants | TLR Agonists | LNP Systems |
| Cardiovascular RNAi | siRNA (e.g., PCSK9) | ApoB, GalNAc | siRNA-Liposomes |
| CNS Disorders | Enzymes / RNA | Transferrin, RGD | Brain-Penetrant Liposomes |
Litchlab is a specialized CDMO partner dedicated to the development and production of liposomal and nanoparticle-based drug delivery systems. From structure-driven lipid design to GMP manufacturing and regulatory support, Litchlab delivers an integrated platform to enable next-generation precision therapies.
In an era where precision medicine leads the future of therapeutics, Litchlab is redefining how delivery systems are designed, manufactured, and translated—delivering smarter systems, sharper efficacy, and faster clinical impact.
📩 Learn more about Litchlab CDMO solutions: www.litchlab.com
📧 Contact: RD2@litchlab.com