
Advancing Drug Delivery Technologies for Precision Medicine and Industrialization
In the development and commercialization of innovative therapeutics, drug delivery systems play a crucial role in determining stability, bioavailability, targeting efficiency, and therapeutic outcomes. With the rapid advancements in mRNA therapies, RNA interference (RNAi), gene editing, immuno-oncology, and long-acting formulations, pharmaceutical companies worldwide are actively seeking more efficient, precise, and scalable drug delivery solutions.
As a global leader in nanomedicine and drug delivery technologies, Litchlab specializes in liposomes, lipid nanoparticles (LNPs), polymeric nanoparticles (PNPs), microspheres, and exosome-based delivery platforms. Our cutting-edge technologies provide end-to-end solutions, from early-stage development to GMP manufacturing, accelerating the translation of RNA therapeutics, gene therapies, cancer treatments, vaccines, and sustained-release formulations into commercial success.
Litchlab’s Core Technological Advantages
Technology Platform | Litchlab Solution | Key Advantages | Clinical Applications |
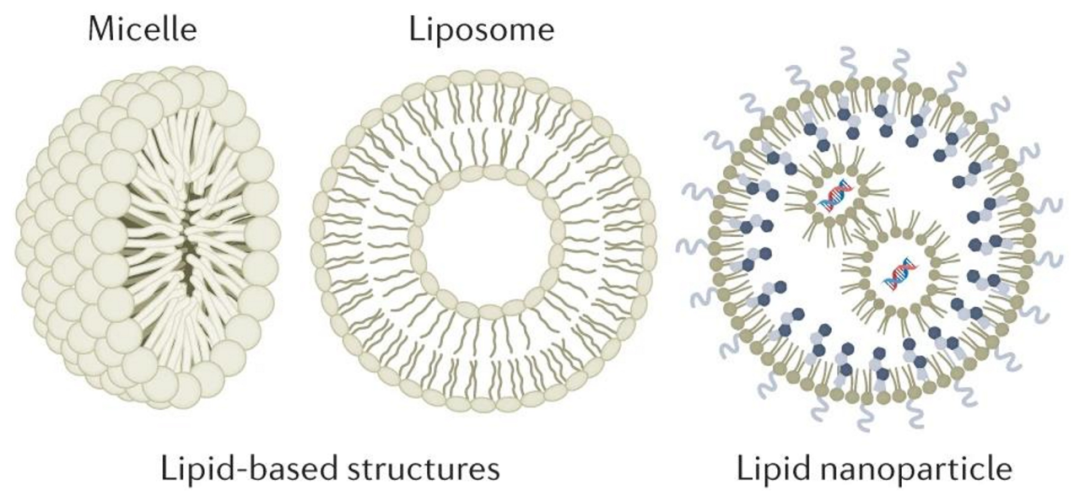
Lipid Nanoparticles (LNPs) | High-efficiency delivery of mRNA, siRNA, CRISPR, optimizing nucleic acid stability | Precision targeting, reduced immunogenicity, scalable manufacturing | mRNA vaccines, RNA therapeutics, gene editing |
Liposomes | Targeted modification, PEGylation for extended circulation time | Enhanced bioavailability, improved tissue penetration | Oncology, vaccines, antibiotics |
Polymeric Nanoparticles (PNPs) | Controlled-release, multilayer design for enhanced stability | Improved local drug concentration, increased bioavailability | Targeted cancer therapy, neurodegenerative diseases |
Microsphere Drug Delivery | Long-acting release formulations (1–6 months) | Reduced dosing frequency, improved patient adherence | Diabetes, psychiatric disorders, chronic inflammation |
Exosome-Based Delivery | Cell-specific targeting for RNA and protein therapeutics | Low immunogenicity, natural biocompatibility | Personalized medicine, immune modulation |
Litchlab’s Full-Spectrum Solutions for Pharmaceutical Companies
1. Formulation Optimization and Nanocarrier Development
Using supercritical fluid technology, microfluidics, solvent evaporation, spray drying, and double emulsification techniques, Litchlab tailors drug delivery carriers based on the physicochemical properties of active pharmaceutical ingredients (APIs)—ensuring precise control of particle size (10–200nm), encapsulation efficiency, and release kinetics, ultimately enhancing stability, cellular uptake, and biodistribution.
2. GMP-Grade Scale-Up and Commercial Manufacturing
Litchlab operates cGMP-compliant facilities adhering to FDA, EMA, and NMPA standards, implementing high-pressure extrusion, microfluidics, ultrasonication, and spray drying technologies to enable seamless transition from small-scale research to commercial production, ensuring batch-to-batch consistency and product stability.
3. Targeted Delivery for Enhanced Precision
Litchlab employs PEGylation, antibody conjugation, aptamer modification, and pH/enzyme-responsive designs to enhance targeting specificity, reduce off-target effects, and improve therapeutic index (TI).
4. CMC Development and Regulatory Support for IND Submission
Our comprehensive CMC (Chemistry, Manufacturing, and Controls) services encompass formulation development, stability testing, quality control, and pharmacokinetic profiling, supporting regulatory submissions worldwide (IND, NDA, BLA) to accelerate clinical translation and market entry.
Case Study: Litchlab’s Advanced RNA Therapeutic Delivery Solutions
RNA-based therapies (mRNA, siRNA, ASO) hold immense potential for oncology, genetic disorders, and infectious diseases. However, their development faces critical challenges:
· RNA instability leading to rapid degradation in vivo
· Poor cellular uptake and lack of tissue specificity
· High immunogenicity causing adverse immune responses
Litchlab leverages LNP and exosome-based delivery technologies to address these challenges:
✅ Optimized LNP formulations for enhanced RNA stability, improving protein expression efficiency
✅ Targeted surface modifications for selective cellular uptake, ensuring precise biodistribution
✅ Immunogenicity reduction strategies to improve safety profiles
✅ GMP-compliant scalable production, meeting global regulatory standards
Future Outlook: Partnering with Global Pharma to Drive Precision Medicine Commercialization
As mRNA vaccines, gene editing, RNAi therapies, and cell & gene therapies continue to reshape the pharmaceutical landscape, advanced drug delivery technologies will be the key differentiator. Litchlab remains at the forefront of nanomedicine innovation, collaborating with pharmaceutical companies worldwide to accelerate the commercialization of next-generation therapeutics.
For more information, please feel free to contact us at:
E-Mail:RD2@Litchlab.com