
Global pharmaceutical leader AstraZeneca has recently announced a licensing agreement with Nanoform, a Finland-based pharmaceutical technology company, to integrate supercritical fluid (SCF) nanotechnology for drug crystallization and optimization at the nanoscale. This cutting-edge technology demonstrates enormous potential in enhancing drug solubility and bioavailability, providing innovative solutions for poorly soluble drugs.
As a global leader in liposomal and nanomedicine drug delivery technologies, Litchlab is actively integrating SCF nanotechnology with its liposomal drug delivery platform to improve the stability and efficacy of RNA therapeutics, oncology drugs, and vaccines.
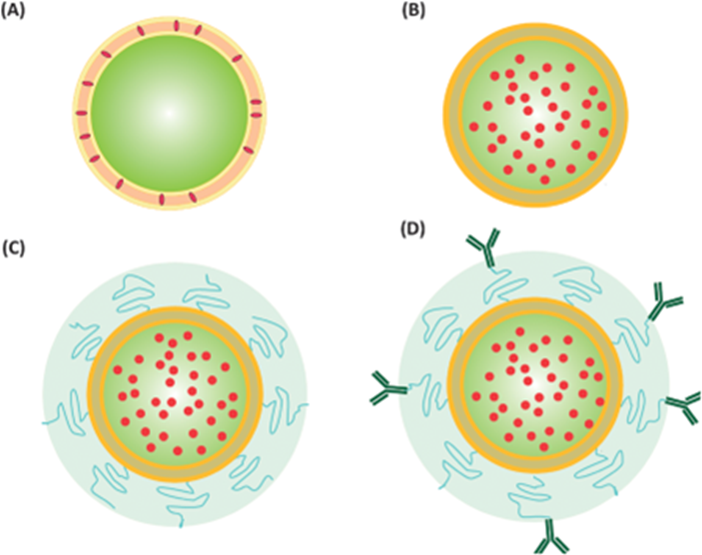
Compared to traditional small-molecule drugs, liposomes have emerged as a core technology in nanomedicine due to their biocompatibility, high drug encapsulation efficiency, and controlled release properties. By combining supercritical nanotechnology, Litchlab is further optimizing liposomal drug delivery systems to advance RNA therapeutics, targeted cancer treatments, and vaccine development.
Technical Feature | Litchlab’s Solution | Clinical Applications |
Supercritical Liposome Preparation | Utilizes SCF technology for highly uniform and stable nanoparticles | mRNA delivery, poorly soluble APIs |
Precision Particle Size Control (50–200 nm) | Enhances cellular uptake and improves biodistribution | siRNA, mRNA, and vaccine delivery |
High Drug Encapsulation Efficiency | Optimized lipid compositions for improved stability of siRNA/mRNA therapeutics | Gene therapy, cancer immunotherapy |
PEGylated Liposomes | Enhances circulation time and improves targeted delivery | Long-circulating RNA drugs, targeted cancer therapies |
Targeted Functionalization | Antibody and aptamer modifications for tumor-specific delivery | Precision oncology, personalized medicine |
✅ mRNA Vaccine Delivery: Optimized LNPs for higher translation efficiency & enhanced immunogenicity
✅ siRNA Targeted Delivery: Increased stability and gene silencing efficiency
✅ ASO (Antisense Oligonucleotide) Delivery: Improved bioavailability, ideal for neurodegenerative diseases
✅ Liposomal Chemotherapy (e.g., Doxorubicin Liposomes): Reduced toxicity, improved efficacy
✅ siRNA + Small Molecule Co-Delivery: Gene regulation for enhanced treatment outcomes
✅ Immunotherapy Nanocarriers: Enhanced PD-1/PD-L1 checkpoint inhibitor efficiency
✅ mRNA Vaccines (e.g., COVID-19, Influenza): Stronger immune response, reduced degradation
✅ Protein Vaccine Liposomal Delivery: Enhanced antigen presentation & immune protection
✅ CRISPR/Cas9 Delivery for Gene Editing: Higher editing precision, reduced off-target effects
✅ Liposomal Sustained-Release Formulations: Long-acting drug delivery for improved patient compliance
Litchlab provides an end-to-end solution, from early-stage formulation development to GMP production, accelerating the transition of nanomedicine drug delivery platforms from lab to commercialization.
Technology Module | Litchlab’s Solution |
Liposomal Drug Delivery Platform | Optimized formulations for enhanced RNA therapeutic efficiency |
Scalable Production Technology | Microfluidics, high-pressure extrusion for GMP-grade manufacturing |
Personalized Targeting | Antibody and aptamer-based targeting for precision medicine |
Stability Optimization | Improved stability for RNA drugs, monoclonal antibodies (mAbs), and biologics |
Nanoparticle Size Control | 50–200 nm precise tuning for better tissue penetration |
Future Outlook
The AstraZeneca-Nanoform collaboration marks a significant advancement in supercritical nanotechnology for poorly soluble drug delivery. Meanwhile, Litchlab is pioneering innovations in liposomal nanotechnology, revolutionizing RNA therapeutics, cancer treatments, and vaccine delivery. Moving forward, Litchlab will continue to partner with leading pharmaceutical companies to accelerate nanomedicine commercialization and bring next-generation therapeutics to patients worldwide.
For more information, please feel free to contact us at:
E-Mail:RD2@Litchlab.com