
mRNA Vaccines and Lipid Nanoparticle Lyophilization Technology: Breakthroughs in Innovative Delivery Systems in Vaccine Development
Driven by the COVID-19 pandemic, mRNA vaccines and lipid nanoparticle (LNP) delivery systems have become key technologies in vaccine development. As a delivery system, lipid nanoparticles not only effectively protect the stability of mRNA but also play a crucial role in enhancing delivery efficiency and improving immunogenic safety. Our company leverages advanced lipid nanoparticle delivery and lyophilization technologies to enhance the application value and efficacy of vaccines and drugs, while providing integrated solutions to overcome storage and transportation challenges.
Core Design Factors of mRNA-LNPs
The development of mRNA-LNPs involves multiple aspects, including optimization of the mRNA sequence, design of LNP formulations, and improvements in storage conditions. In the design process, the development team needs to carefully consider the following factors:
1. mRNA Sequence Design and Modification
Sequence Optimization: The expression level of an mRNA sequence is closely related to nucleotide modifications. Unmodified nucleotides may be recognized by intracellular RNA sensors, triggering an innate immune response and leading to rapid clearance of the mRNA. Therefore, to enhance protein expression, mRNA sequences are often modified, such as introducing pseudouridine or other modifications to reduce immunogenicity and improve mRNA stability.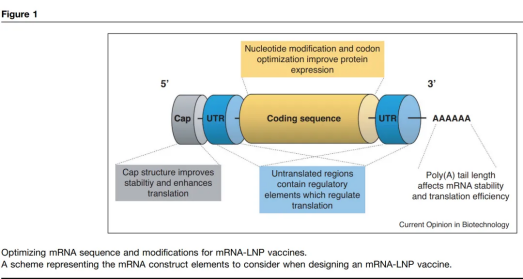
Cap Structure: Before translation, mRNA requires a cap structure to enhance translation efficiency and intracellular stability. Modern mRNA-LNP vaccines typically use technologies like Anti-Reverse Cap Analog (ARCA) to ensure the correct orientation of the cap, further improving protein expression efficiency.
UTR Optimization: The untranslated regions (UTRs) also play a key role in mRNA stability and translation efficiency. Optimizing the 5' and 3' UTRs can significantly increase the mRNA half-life, reduce intracellular degradation, and improve protein expression.
2. LNP Formulation Design and Optimization
Cationic Lipid Selection: The cationic lipids in LNPs are key components, influencing the stability of mRNA-LNPs, their delivery efficiency, and the immune response. Ideal cationic lipids should be biodegradable in the body, have low toxicity, and adapt to changes in pH, improving cell uptake and release.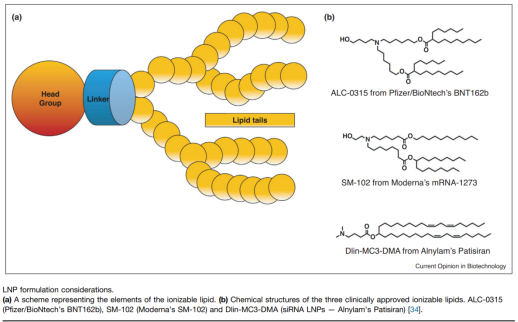
PEG Lipids: PEG (Polyethylene glycol) in LNPs helps provide stability, control nanoparticle size, and prevent aggregation. However, the proportion of PEG needs to be carefully controlled to avoid hindering mRNA uptake into cells. Some recent studies have also explored alternatives to PEG to reduce potential immune responses.
Lipid Tail and Junction Region: The hydrophobicity and unsaturation of lipid tails determine the structure and delivery efficiency of LNPs. By introducing degradable ester bonds into lipid tails, drug pharmacokinetics can be improved, and long-term toxicity risks can be reduced.
3. Storage Conditions and Cryoprotection
Optimization of Freezing Storage: The ultra-low-temperature storage requirements of mRNA-LNP vaccines pose significant challenges in global distribution and supply. Vaccines like BNT162b2 and mRNA-1273 require storage at -80°C and -20°C, respectively, which places high demands on transportation and storage conditions. Recent studies have found that adding cryoprotectants such as sucrose or trehalose to LNP formulations can stabilize the mRNA structure, improving storage time and efficacy.
Lyophilization Technology: For long-term storage of mRNA vaccines, lyophilization (freeze-drying) is a promising method. It can not only extend the shelf life of vaccines but also reduce the cost of ultra-low-temperature storage. However, due to the high cost and complexity of the lyophilization process, further research is needed to ensure that this process does not negatively affect mRNA activity or LNP stability. Our company’s innovative lyophilization technology addresses the storage challenge of mRNA-LNPs by removing moisture, allowing lipid formulations to remain stable at room temperature and reducing cold chain transportation costs. Lyophilization effectively prevents moisture from affecting vaccine stability, preserving vaccine efficacy.
Improvement Strategies for mRNA-LNPs
1. Development of New Cationic Lipids
With the expanding clinical application of mRNA vaccines, the development of low-toxicity, high-efficiency cationic lipids will continue to be a focus in the optimization of mRNA-LNP technology. Studies have shown that modifying the structure to increase the unsaturation of lipid tails or introducing biodegradable ester bonds can improve the stability of LNPs in the body, thereby enhancing protein expression. Our company focuses on the development of novel cationic lipids, using structural modifications and the introduction of degradable ester bonds to improve LNP stability and cell uptake efficiency, optimizing mRNA expression.
2. New Cold Chain Storage Solutions
In response to the ultra-low-temperature storage requirements of mRNA vaccines, both academia and industry are working together to explore new cold chain solutions. By removing moisture with lyophilization, lipid nanoparticles can be stored stably at room temperature, avoiding the significant costs and complexities associated with long-term cold chain transportation. Additionally, lyophilization ensures that mRNA-LNPs are protected from moisture during storage and transportation, thereby better preserving the stability and efficacy of vaccines.
Conclusion
The mRNA-LNP technology platform has demonstrated new potential and application prospects in vaccine development. Our lipid nanoparticle lyophilization technology ensures both stability and long-term storage through precise temperature control, unique formulation design, and efficient vacuum drying. Moving forward, we will continue to invest in research and development to promote the further advancement of lipid nanoparticles and mRNA vaccines, helping to address global epidemic challenges. The potential applications of the mRNA-LNP platform will expand, possibly ushering in a new era for disease prevention and treatment.